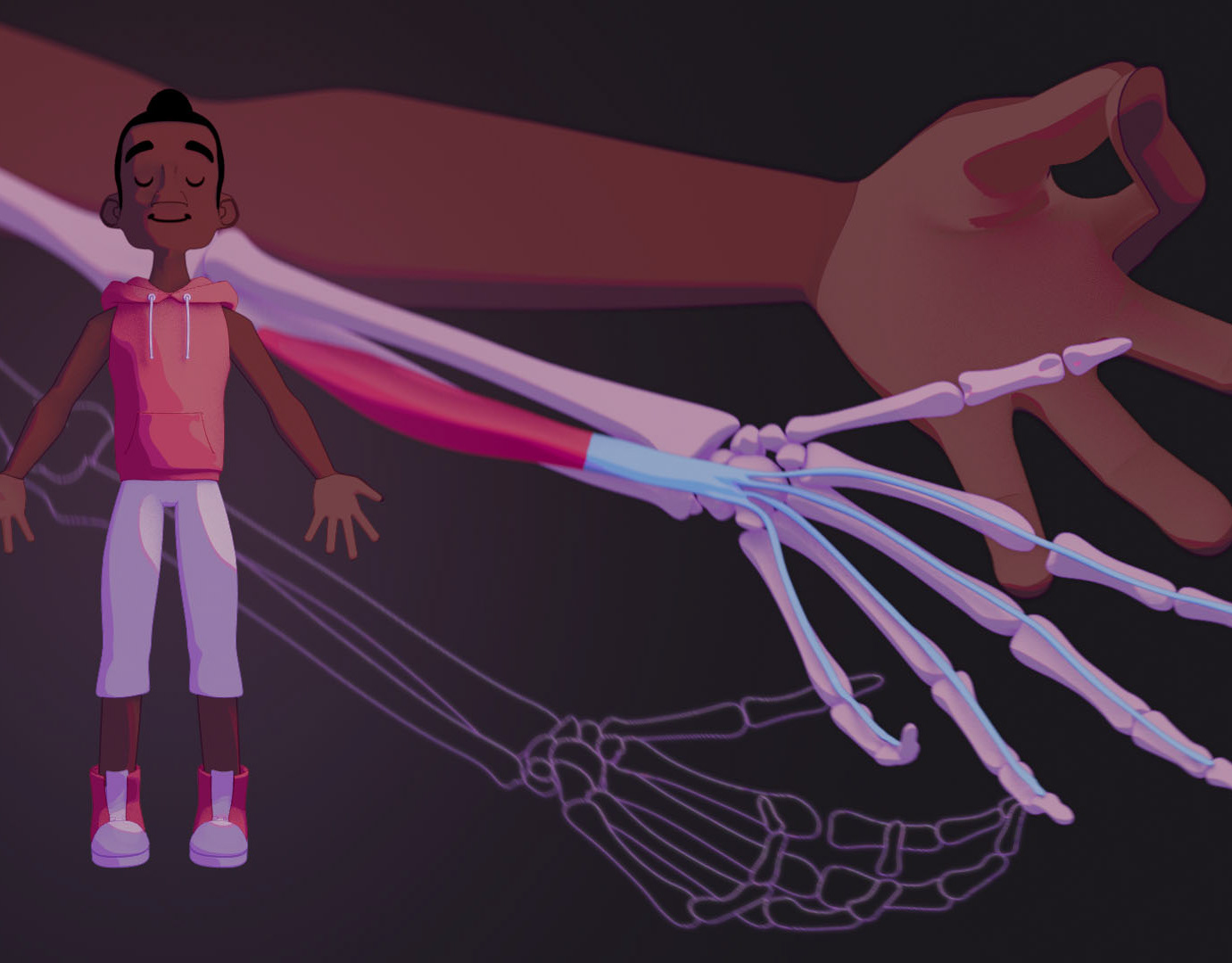
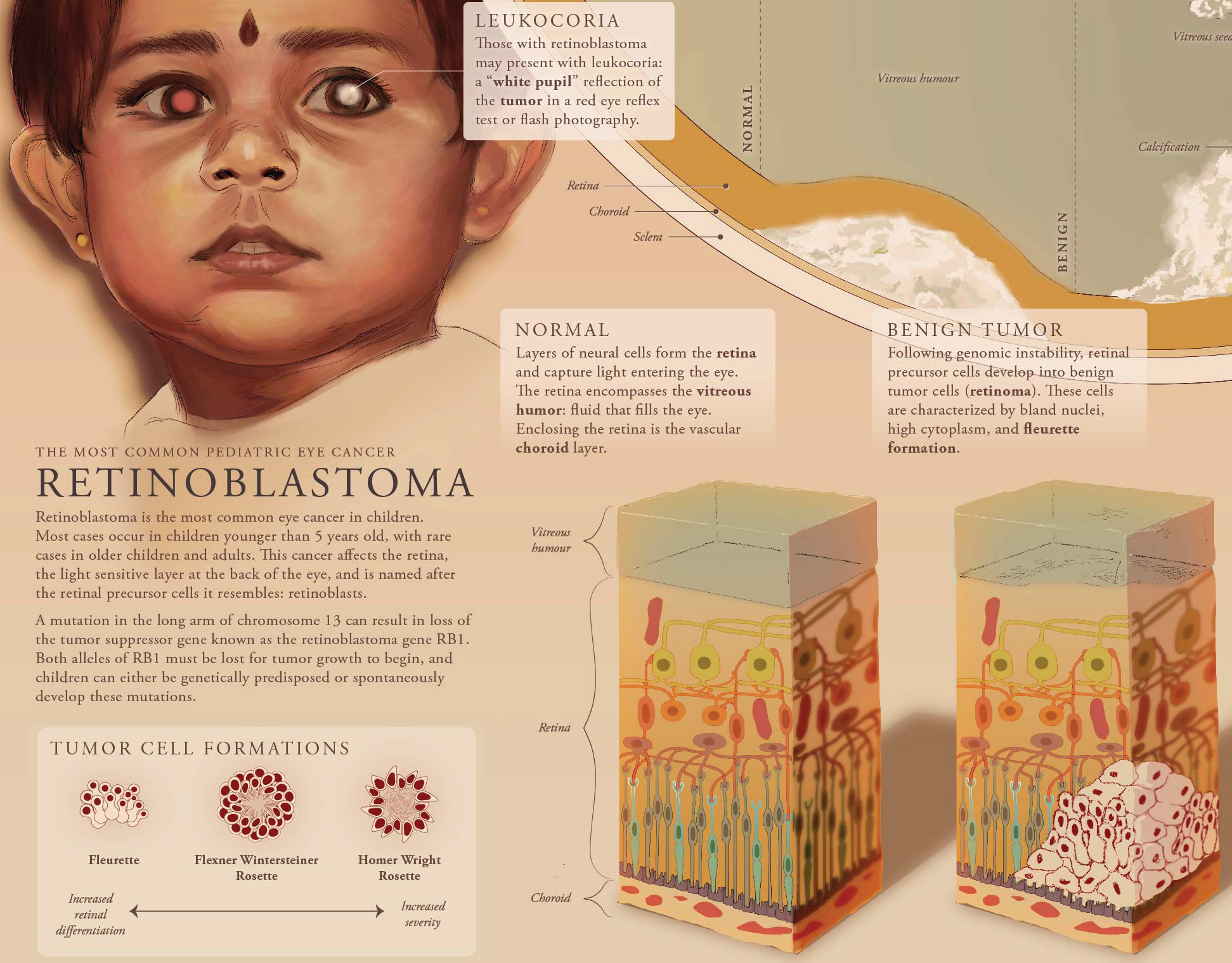
This molecular visualization informs a general audience about the science of antifreeze proteins (AFPs). The diversity of life that produces AFPs is shown with examples of a fish, insect, fungus, and plant.
Audience: General Public
Client: Prof. Derek Ng
Software: Photoshop, Illustrator, Chimera, VMD, Maya
Medium: 2-Page Magazine Spread
Research & Media Audit
Early on, I focused my research on structures, functions, and applications related to AFPs. At this stage, I was interested in how AFPs provide insight into storing human organs for transplantation at low temperatures without being damaged.
I also conducted a media audit of existing visualizations exploring AFPs and identified key gaps in communication for a general audience.
Composition
Using the initial research, I sketched out compositions based on different communication goals outlining AFPs' function and applications.
Discussions with Prof. Ng revealed that while each subsection had a clear purpose, these options were too uniform; at a glance, the main purpose was not clear.
Layout
To incorprate feedback from the initial sketches, I redid the composition to make two main ideas clear: the mechanism as well as diversity of AFPs. I used PDB data to create placeholder assets and developed a layout draft.
In another round of feedback, the combination of volume and ribbon representations for the AFPs confused some to think that the proteins were bound to some molecule. I then adjusted the placeholder assets to better showcase the ice-binding sites, as shown in the finalized layout.
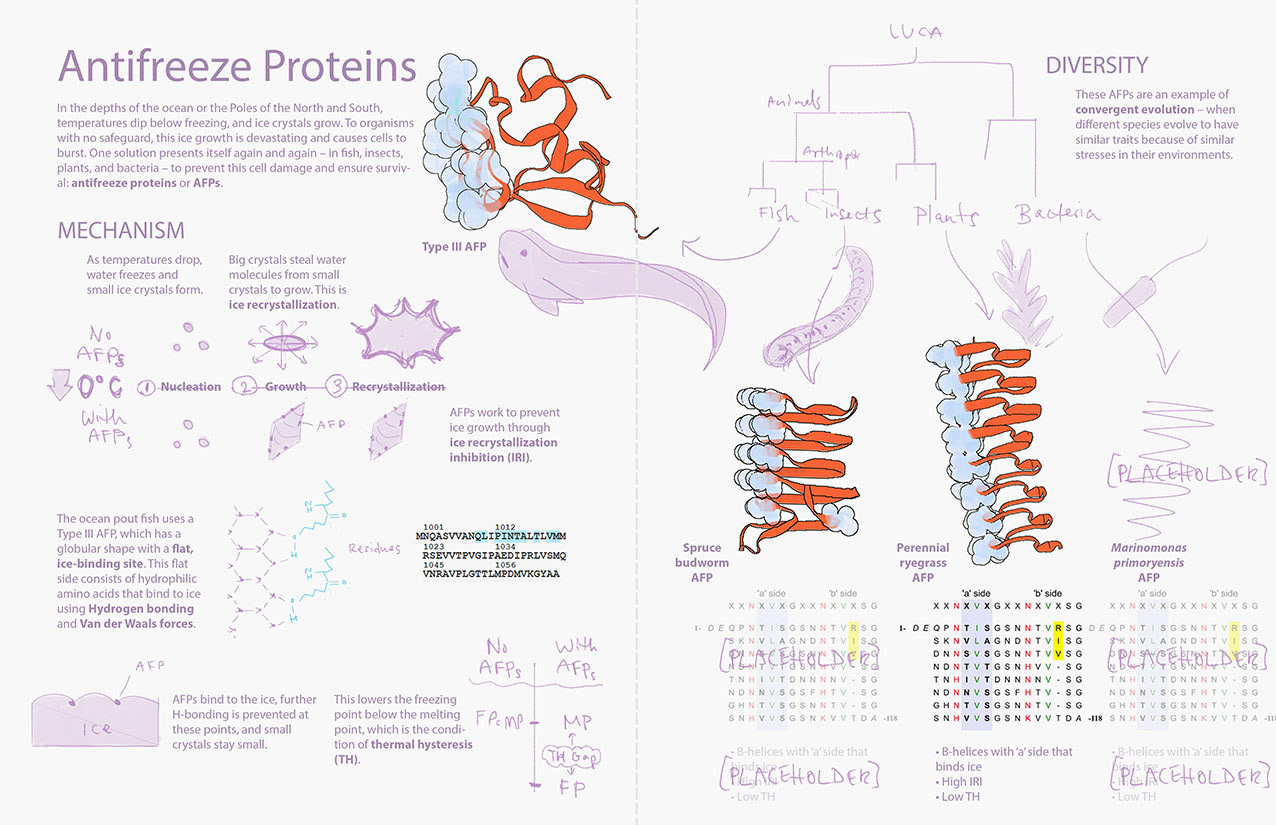
Layout Draft
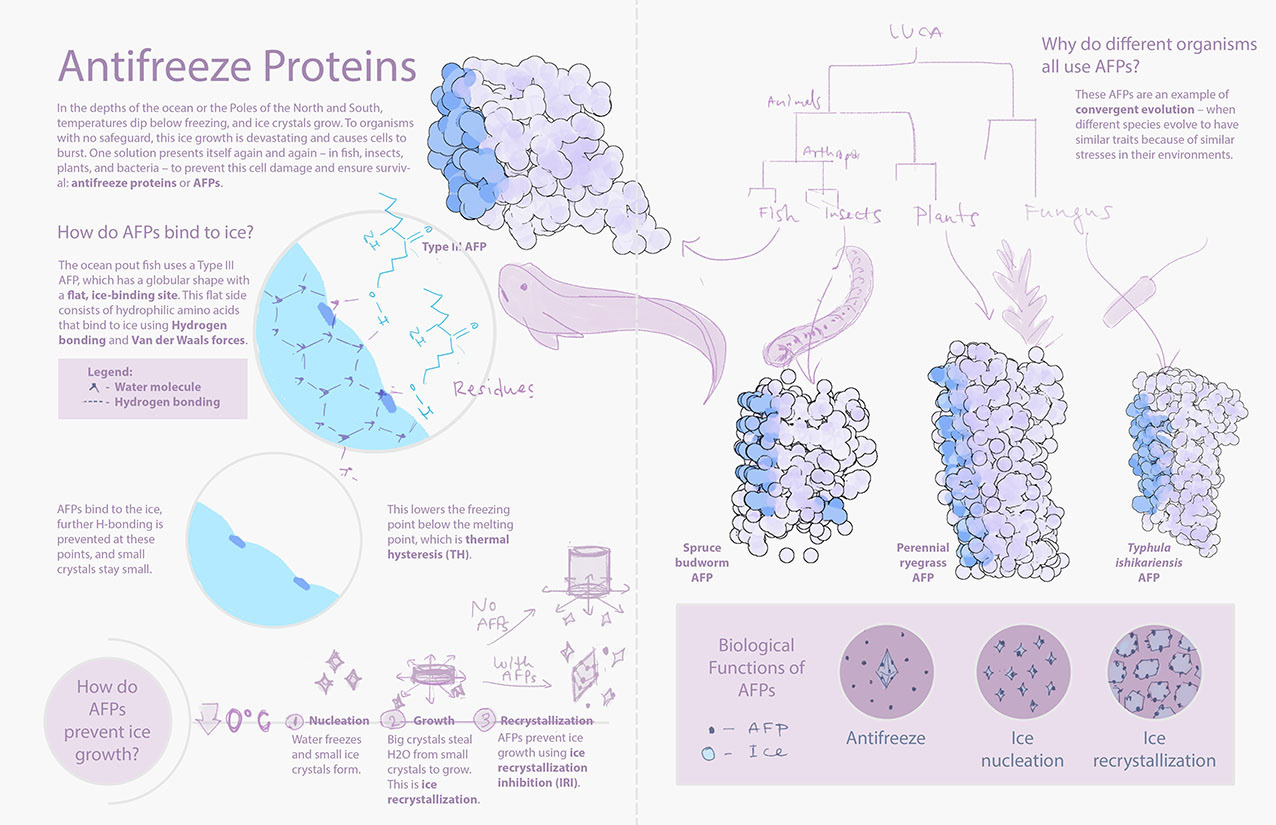
Finalized Layout
References
1. Antson, A. A., Smith, D. J., Roper, D. I., Lewis, S., Caves, L. S., Verma, C. S., Buckley, S. L., Lillford, P. J., & Hubbard, R. E. (2001). Understanding the mechanism of ice binding by type III antifreeze proteins. Journal of molecular biology, 305(4), 875–889. https://doi.org/10.1006/jmbi.2000.4336
2. Baskaran, A., Kaari, M., Venugopal, G., Manikkam, R., Joseph, J., & Bhaskar, P. V. (2021). Anti freeze proteins (Afp): Properties, sources and applications – A review. International Journal of Biological Macromolecules, 189, 292–305. https://doi.org/10.1016/j.ijbiomac.2021.08.105
3. Chapman, A. (2009) Lolium perenne [Image]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Lolium_perenne.jpg
4. Cheng, J., Hanada, Y., Miura, A., Tsuda, S., & Kondo, H. (2016). Hydrophobic ice-binding sites confer hyperactivity of an antifreeze protein from a snow mold fungus. Biochemical Journal, 473(21), 4011–4026. https://doi.org/10.1042/BCJ20160543
5. Davies, P. L. (2014). Ice-binding proteins: a remarkable diversity of structures for stopping and starting ice growth. Trends in Biochemical Sciences (Amsterdam. Regular Ed.), 39(11), 548–555. https://doi.org/10.1016/j.tibs.2014.09.005
6. Dewey, J. E. (2001) Choristoneura fumiferana larva [Image]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Choristoneura_fumiferana_larva.jpg
7. Gharib, G., Saeidiharzand, S., Sadaghiani, A. K., & Koşar, A. (2021). Antifreeze Proteins: A Tale of Evolution From Origin to Energy Applications. Frontiers in Bioengineering and Biotechnology, 9, 770588–770588. https://doi.org/10.3389/fbioe.2021.770588
8. Keats, D. (2011) Ocean pout, Newfoundland, Canada [Image]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Ocean_pout,_Newfoundland,_Canada.jpg
9. Kuiper, M.J., Davies, P. L., Gagné, S. M., Graether, S. P., Jia, Z., Sykes, B. D., & Walker, V. K. (2000). β-Helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature (London), 406(6793), 325–328. https://doi.org/10.1038/35018610
10. Leinala, E. K., Davies, P. L., Doucet, D., Tyshenko, M. G., Walker, V. K., & Jia, Z. (2002). A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights. The Journal of biological chemistry, 277(36), 33349–33352. https://doi.org/10.1074/jbc.M205575200
11. Matsumoto, N., Hoshino, T., Yamada, G., Kawakami, A., & Takada-Hoshino, Y. (2010). Sclerotia of Typhula ishikariensis biotype B (Typhulaceae) from archaeological sites (4000 to 400 BP) in Hokkaido, northern Japan. American Journal of Botany, 97(3), 433–437. https://doi.org/10.3732/ajb.0900133
12. Middleton, A. J., Marshall, C. B., Faucher, F., Bar-Dolev, M., Braslavsky, I., Campbell, R. L., Walker, V. K., & Davies, P. L. (2012). Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. Journal of molecular biology, 416(5), 713–724. https://doi.org/10.1016/j.jmb.2012.01.032
13. Tas, Sampaio-Pinto, V., Wennekes, T., van Laake, L. W., & Voets, I. K. (2021). From the freezer to the clinic: Antifreeze proteins in the preservation of cells, tissues, and organs. EMBO Reports, 22(3), e52162–e52162. https://doi.org/10.15252/embr.202052162
14. Tyshenko, M.G., Doucet, D., & Walker, V. K. (2005). Analysis of antifreeze proteins within spruce budworm sister species. Insect Molecular Biology, 14(3), 319–326. https://doi.org/10.1111/j.1365-2583.2005.00562.x
1. Antson, A. A., Smith, D. J., Roper, D. I., Lewis, S., Caves, L. S., Verma, C. S., Buckley, S. L., Lillford, P. J., & Hubbard, R. E. (2001). Understanding the mechanism of ice binding by type III antifreeze proteins. Journal of molecular biology, 305(4), 875–889. https://doi.org/10.1006/jmbi.2000.4336
2. Baskaran, A., Kaari, M., Venugopal, G., Manikkam, R., Joseph, J., & Bhaskar, P. V. (2021). Anti freeze proteins (Afp): Properties, sources and applications – A review. International Journal of Biological Macromolecules, 189, 292–305. https://doi.org/10.1016/j.ijbiomac.2021.08.105
3. Chapman, A. (2009) Lolium perenne [Image]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Lolium_perenne.jpg
4. Cheng, J., Hanada, Y., Miura, A., Tsuda, S., & Kondo, H. (2016). Hydrophobic ice-binding sites confer hyperactivity of an antifreeze protein from a snow mold fungus. Biochemical Journal, 473(21), 4011–4026. https://doi.org/10.1042/BCJ20160543
5. Davies, P. L. (2014). Ice-binding proteins: a remarkable diversity of structures for stopping and starting ice growth. Trends in Biochemical Sciences (Amsterdam. Regular Ed.), 39(11), 548–555. https://doi.org/10.1016/j.tibs.2014.09.005
6. Dewey, J. E. (2001) Choristoneura fumiferana larva [Image]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Choristoneura_fumiferana_larva.jpg
7. Gharib, G., Saeidiharzand, S., Sadaghiani, A. K., & Koşar, A. (2021). Antifreeze Proteins: A Tale of Evolution From Origin to Energy Applications. Frontiers in Bioengineering and Biotechnology, 9, 770588–770588. https://doi.org/10.3389/fbioe.2021.770588
8. Keats, D. (2011) Ocean pout, Newfoundland, Canada [Image]. Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Ocean_pout,_Newfoundland,_Canada.jpg
9. Kuiper, M.J., Davies, P. L., Gagné, S. M., Graether, S. P., Jia, Z., Sykes, B. D., & Walker, V. K. (2000). β-Helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature (London), 406(6793), 325–328. https://doi.org/10.1038/35018610
10. Leinala, E. K., Davies, P. L., Doucet, D., Tyshenko, M. G., Walker, V. K., & Jia, Z. (2002). A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights. The Journal of biological chemistry, 277(36), 33349–33352. https://doi.org/10.1074/jbc.M205575200
11. Matsumoto, N., Hoshino, T., Yamada, G., Kawakami, A., & Takada-Hoshino, Y. (2010). Sclerotia of Typhula ishikariensis biotype B (Typhulaceae) from archaeological sites (4000 to 400 BP) in Hokkaido, northern Japan. American Journal of Botany, 97(3), 433–437. https://doi.org/10.3732/ajb.0900133
12. Middleton, A. J., Marshall, C. B., Faucher, F., Bar-Dolev, M., Braslavsky, I., Campbell, R. L., Walker, V. K., & Davies, P. L. (2012). Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. Journal of molecular biology, 416(5), 713–724. https://doi.org/10.1016/j.jmb.2012.01.032
13. Tas, Sampaio-Pinto, V., Wennekes, T., van Laake, L. W., & Voets, I. K. (2021). From the freezer to the clinic: Antifreeze proteins in the preservation of cells, tissues, and organs. EMBO Reports, 22(3), e52162–e52162. https://doi.org/10.15252/embr.202052162
14. Tyshenko, M.G., Doucet, D., & Walker, V. K. (2005). Analysis of antifreeze proteins within spruce budworm sister species. Insect Molecular Biology, 14(3), 319–326. https://doi.org/10.1111/j.1365-2583.2005.00562.x